eMDR Process Overview
The Food and Drug Administration (FDA) has initiated the Electronic Medical Device Reporting (eMDR) project. This project allows manufacturers, importers, and user facilities to submit their medical device adverse event reports electronically.
The eMDRs are submitted through the Electronic Submissions Gateway (ESG), an agency-wide solution for accepting electronic regulatory submissions. The ESG enables regulatory information to be securely submitted for review using a single point of entry.
|
|
To set up eMDRs on your Windchill Risk and Reliability installation, see Getting Started with eMDRs.
|
Options for Electronic Submission
The FDA supports two options for electronic submission:
• High volume batch reporting
◦ Submitting a Health Level 7 (HL7) Individual Case Safety Report (ICSR) form
◦ Using B2B to submit XML files through the FDA Gateway
• Low volume single reporting
◦ Submitting a 3500Z form
◦ Using WebTrader to submit ZIP files through the ESG
eMDR Workflow
Windchill Risk and Reliability has created an online version of the high volume Health Level Seven (HL7) Individual Case Safety Report (ICSR) form. After you complete and submit the form, Windchill Customer Experience Management translates the input into the 3500A format for the FDA using standard XML. Windchill Customer Experience Management then forwards the form to the FDA.
During the submission process, Windchill Customer Experience Management receives three separate acknowledgements from the ESG about the status of an eMDR. Windchill Customer Experience Management automatically processes incoming acknowledgements and shows the data from each acknowledgement in the submission table for each eMDR. If the submission fails during any of the three acknowledgements, you must make corrections and resubmit.
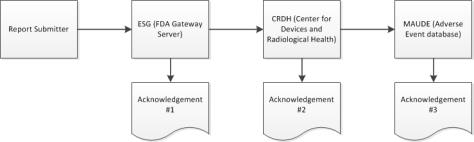
1. Submit your eMDR. For instructions, see Creating or Updating an eMDR.
◦ Delivery and processing time depends on the overall size of your submission; larger submissions take longer to be delivered and processed.
◦ You must have a electronic signature to make submissions.
◦ Submission filenames can include only one period, which is used to indicate the file type extension (for example, 555.xml or 555.pdf).
2. When your submission reaches the ESG, you should receive acknowledgment #1 quickly after you submit the eMDR, unless the ESG is down for maintenance.
◦ Check the status on the ESG web site at http://www.fda.gov/Food/GuidanceRegulation/FoodFacilityRegistration/ucm161883.htm.
◦ If you have further questions, contact the ESG at esgreg@gnsi.com.
3. The eMDR is automatically routed from the ESG to the Center for Devices and Radiological Health (CDRH). When your submission reaches the CDRH, you should receive acknowledgment #2.
The FDA system can take up to 48 hours before sending the second acknowledgement. If you do not receive a response within that time, contact the ESG at esgreg@gnsi.com with the report number. Do not resubmit the eMDR until the submission is shown as failed in Windchill Customer Experience Management or until the FDA instructs you to do so. For instructions, see Resolving a Delayed eMDR. |
4. When the CDRH validates and loads the submission into the Adverse Event database (MAUDE), you should receive acknowledgment #3. Any errors that occur during validation and loading are noted.
If there are error messages in the third acknowledgement, you must correct the errors and resubmit, beginning with step 1. After you resubmit, you will receive another set of three acknowledgments. The FDA system can take up to 48 hours before sending acknowledgement #2 or #3. If you do not receive a response within that time, send an email to the FDA at emdr@fda.hhs.gov with the report number. Wait 24 hours between contacting the ESG and contacting the FDA. Do not resubmit the eMDR until the submission fails or the FDA instructs you. For instructions, see Resolving a Delayed eMDR. |