Windchill Quality Solutions: eMDR Follow-Ups
Product: Windchill, Windchill Quality Solutions, Windchill Customer Experience Management
Release: 11.0 M030
Benefit
This enhancement makes it easier for customers to comply with the revised standard for eMDR HL7 from the FDA.
Additional Details
To comply with the revised standard for eMDR HL7 from the FDA in June 2015, Windchill has implemented the following changes:
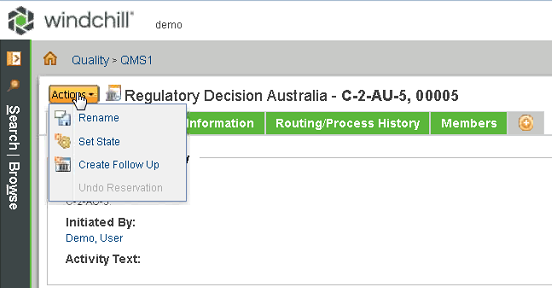
• Previously, you used the Set State function or create a new decision for the report to create a follow-up for Australia, Israel, and Japan reports. You can now select the Create Follow Up action on the report decision page to create a follow-up for these reports.
• Previously, you used the Create Follow Up action to create a follow-up for the FDA eMDR report, the Health Canada Vigilance report, and the European Vigilance report, but the function was difficult to locate. The Create Follow Up action is now available on the report decision page for these reports to make this function easier to access.
• When you create a follow-up report, the system automatically completes the Additional Information Aware Date field with the current date if no value exists in the field. If there is already a date in the field, the system does not change it.
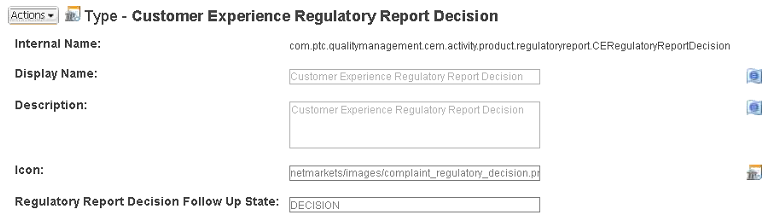
• The default states for newly created follow-up reports are controlled by the Regulatory Report Decision Follow Up State attribute on the Customer Experience Regulatory Report Decision type in the Type and Attribute Management utility. For new installations, the default state of the attribute is Decision. For updates and upgrades, the default state is Creation.
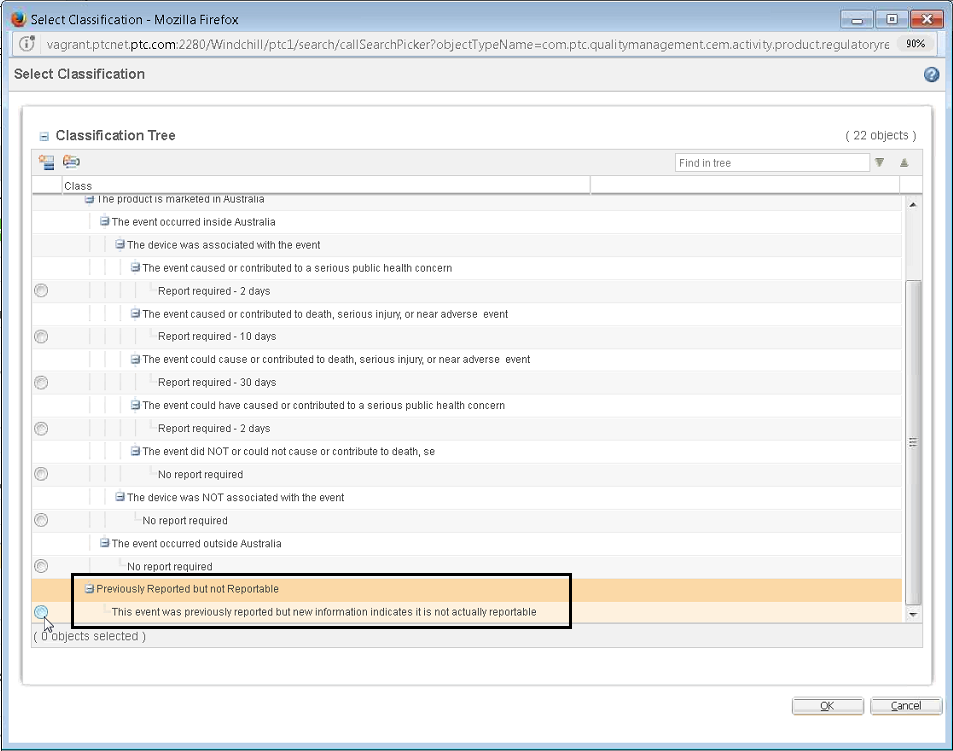
• A new actionable Previously Reported but not Reportable node has been added to the classification tree for each regulatory report. Select this node when you are voiding the previous report because it should not have been sent.
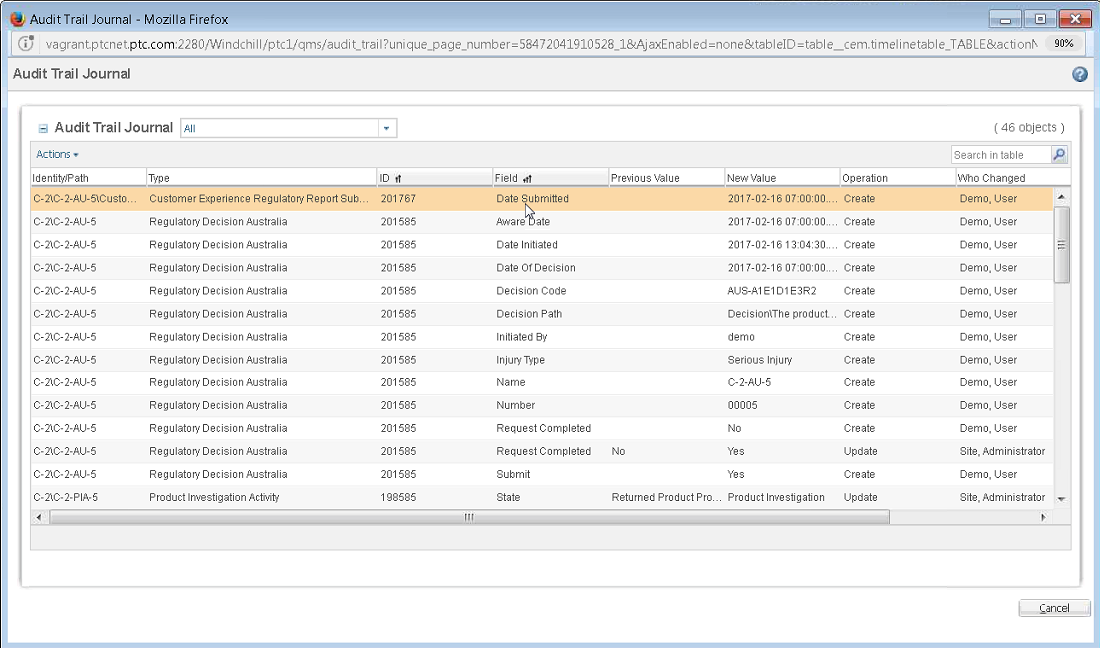
• The CERegulatoryReportSubmissionExternal object has been added to history generation and audit trail reports. This allows submission dates to be captured in the audit trail.
• For new installations, the Reportable field on reports has been changed to Submit.
Related Information